ST332 & ST409 - Medical Statistics Practical 5 - Meta-Analysis
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
ST332 & ST409 - Medical Statistics
Practical 5 - Meta-Analysis
Marson et al. (2001) reported on a systematic review of four anti-epilepsy drugs which had been introduced in the late 20th century. The article is on the Moodle. Four randomised controlled clinical trials had been published which compared the effectiveness of Levetiracetam (Lev) with placebo (P) as an additional treatment for patients with drug resistant localization re-lated epilepsy. The doses of levetiracetam prescribed were 1g, 2g, 3g or 4g. However, no trial included all four doses, see Table 1 of Marson et al. (2001) for a summary of the type of trial, number of patients, and doses prescribed. Patients are said to respond to treatment if their seizure rate at the end of the trial is less than half their rate just before the trial. The results are summarised in the following 2 2 tables:
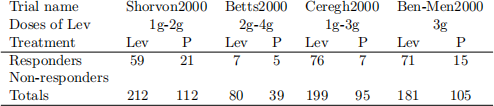
1. For each trial calculate the percent of responders on Lev and placebo. Also estimate the odds ratios for each trial. Comment on the percent-ages and odds ratios.
2. Pool the data into a single 2 × 2 table (Regard the data for all the patients as if they had been in a single trial). Estimate the resulting percentage of responders on Lev and placebo, and the log-odds ra-tio. What do you conclude? Why might this not be an appropriate analysis?
3. Calculate the inverse variance weighted fixed effect estimate of the odds ratio: Compare it with the naive pooled estimator. Which of the four trials has the largest weight? Could you have guessed this without calculating the variances?
4. Use the metabin function in the R meta package to check your calcu-lation of the fixed effect model and obtain an estimate for a random effects model. What do you conclude as regards which model is the most appropriate? Use the forest function to get an appropriate Forest Plot.
5. Use the metareg function to explore whether the pooled odds ratio varies with the average dose in each trial.
2024-04-11