MCB 2210 PROBLEM SET 4
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
MCB 2210 PROBLEM SET 4
1. How are proteins imported into mitochondria and chloroplasts?
a. Co-translationally.
b. Imported fully-formed and properly-folded across membrane of the appropriate organelle.
c. Post-translationally.
d. Pre-transcriptionally.
e. b and c are correct.
2. The N-terminal targeting sequence of mitochondrial-matrix proteins is ultimately removed by
a. a chaperone
b. a mitochondrial processing glycosidase
c. mitochondrial processing lipase
d. mitochondrial signal peptidase
e. mitochondrial ATP synthase
3. How do the signal sequences of nuclear-encoded mitochondrial proteins targeting the inner mitochondrial membrane differ from those targeting the mitochondrial matrix?
a. Inner mitochondrial membrane protein signals are all made of alanine.
b. Inner mitochondrial membrane protein signals remain as part of the molecule.
c. Inner mitochondrial membrane protein signals are internal rather than on the end. d. b and c
e. Inner mitochondrial membrane protein signals contain adenine.
4. Which are functions that molecular chaperones perform to help proteins that enter mitochondria?
a. they keep polypeptides unfolded in the cytoplasm
b. they help pull polypeptides into the mitochondrial matrix
c. they help fold mitochondrial proteins once they reach the matrix
d. they direct mitochondrial proteins to the outer mitochondrial membrane e. a, b and c
5. The outer mitochondrial membrane contains a protein import complex, the that includes a(n) and a protein-lined .
a. TOM complex, receptor that recognizes and binds mitochondrial proteins, channel b. TIM complex, receptor that recognizes and binds mitochondrial proteins, channel
c. TOM complex, receptor that recognizes and binds the outer mitochondrial membrane, channel
d. TIM complex, receptor that recognizes and binds the outer mitochondrial membrane, channel
e. importer, receptor that recognizes and binds mitochondrial proteins, channel
6. Which is thought to help power the movement of the mitochondrial targeting signal on a mitochondrial protein through the TIM complex into the mitochondrial matrix?
a. electric potential across the inner mitochondrial membrane acting on the positively charged targeting signal
b. electric potential across the outer mitochondrial membrane acting on the positively charged targeting signal
c. ATP
d. GTP
e. electric potential across the inner mitochondrial membrane acting on the negatively charged targeting signal
7. Put the following in the correct order:
I. Protein is imported through the TIM complex
II. Protein is imported through the TOM complex
III. Fully-folded, mature protein is located in the mitochondrial matrix
IV. Protein is folded by mitochondrial chaperone using ATP
V. Import signal is recognized by receptor protein
a. V, I, II, IV, III
b. II, V, III, I, IV
c. II, III, V, IV, I
d. V, II, I, IV, III
e. none of the above is correct.
8. Proteins that are destined for the thylakoid membrane or the thylakoid lumen must have:
a thylakoid targeting domain
b. a thylakoid lumen domain
c. a transit peptide
d. a stroma-targeting domain
e. a and d
9. Compared to mitochondria, chloroplasts have an extra membrane-enclosed compartment called:
a. Thylakoid
b. Lumen
c. Cytoplasm
d. Stroma
e. Nucleus
10. If you took a gene that is normally present in the mitochondrial genome and codes for an enzyme that works in the matrix, and then inserted the gene into the nucleus of a cell, what would you expect the final destination of the protein to be?
a. ER lumen
b. Nucleus.
c. Secreted.
d. Mitochondrial matrix
e. None of the above
11. If you took a gene for a protein which is normally found in the cytoplasm and added a mitochondrial targeting signal (N-terminal amphipathic helix) to it and expressed the gene in the cell, where would you expect the protein to end up
a. ER lumen
b. Nucleus
c. Cytoplasm
d. Secreted
e. Mitochondrial matrix
12. If you engineered a nuclear-encoded gene for a mitochondrial matrix protein to contain an Asn-X-Ser glycosylation signal and a c-terminal peroxisomal targeting signal, and then inserted the gene into the nucleus of a cell, and examined the expressed protein, the protein could:
a. Be glycosylated and found in the mitochondrial matrix.
b. Be unglycosylated and in the cytoplasm.
c. Be unglycosylated and exported from the cell.
d. Be unglycosylated and found in the mitochondrial matrix.
e. Be glycosylated and found in the peroxisome.
13. Which is FALSE: In order to target a protein to the lysosomes the protein needs to: a. Be made on ribosomes of the rough endoplasmic reticulum
b. Have a net positive charge
c. Have an Asn-X-Ser glycosylation sequence
d. Be maintained in an unfolded state by chaperones until it reaches the lysosome
e. b and d are false.
14. If you engineered a lysosomal membrane protein that uses an internal start transfer sequence to also contain an N-terminal mitochondrial localization signal, where would the engineered protein likely be found when expressed in cells?
a. Nucleus.
b. Cytoplasm.
c. Mitochondria.
d. Lysosomes.
e. None of the above are correct.
15. Which is the FOURTH step of the five listed in lysosomal enzyme sorting?
a. Co-translational addition of an oligosaccharide to the protein.
b. Binding of Man-6-Phosphate to its receptor in the TGN.
c. Removal of the man-6-phosphate signal
d. Transport of the enzyme from the TGN
e. Transport from the ER to the Golgi
16. PTS1- and PTS2-bearing matrix proteins utilize:
a. a common cytosolic receptor.
b. a common translocation machinery on the peroxisomal membrane.
c. a common receptor on the nuclear pore that catalyzes entry into the nucleus via pore targeting sequences.
d. a common receptor protein within the peroxisomal matrix that activates protein processing for PTS1- and PTS2-bearing proteins.
17. Compartments from different parts of the Golgi complex ranging from the cis-most to the
trans-golgi network (TGN) display differences in enzymatic activity. Which item(s) below might be correct descriptions of those differences?
a. They contain different enzymes that add different sugars to the ends of growing carbohydrate chains of glycoproteins.
b. They contain different ribosomes.
c. They contain different polynucleotides that add different sugars to the ends of growing carbohydrate chains of glycoproteins.
d. They contain different enzymes that add different sugars to the ends of growing carbohydrate chains of polynucleotides.
e. a and b
18. What determines the sequence of sugars in the oligosaccharides of a secretory glycoprotein?
a. the sequence of glycosyltransferases in the ER and Golgi complex membranes.
b. the sequence of coat proteins used to form vesicles ER and Golgi complex membranes.
c. the spatial localization of ribosomes in the pathway membranes.
d. the degree of hydrophilicity of the sugars.
e. a and c
19. What has been used to visualize the journey of secretory proteins from the ER to the Golgi complex?
a. The proteins have been tagged with signal peptides.
b. The proteins have been tagged with green fluorescent protein.
c. The proteins have been tagged with RNAs.
d. The proteins have been tagged with Ran-GTP.
e. b and d
20. The movement of cargo vesicles farther away from the ER and toward the Golgi complex can occur along tracks composed of what material?
a. RNA
b. DNA
c. microtubules
d. microfilaments
e. intermediate filaments
21. Which terms might be used to describe the part of the Golgi complex is closest to and receives material from the ER?
a. the cis-most face
b. the CGN
c. the cis Golgi network
d. trans-Golgi network
e. a, b and c
22. Which of the following carbohydrates is not processed in the Golgi complex?
a. glycosaminoglycans of secreted extracellular matrix proteins in animal cells
b. secreted plant cell wall polysaccharides like pectin and hemicellulose
c. the carbohydrates of glycolipids facing the extracellular environment
d. the N-linked carbohydrates of glycoproteins
e. glycogen, used by cells as an internal energy store
23. Which of the models below suggests that the Golgi cisternae are transient structures that form at the cis face and travel through the Golgi complex, moving physically and exiting the organelle at the trans face, while changing during the journey?
a. the cisternal maturation model
b. the cargo carrying model
c. the vesicular transport model
d. the secretory transport model
e. the chemiosmotic model
24. Vesicles that move through the Golgi complex from the cis-cisternae to the trans-cisternae are said to move in a(n) direction.
a. retrograde
b. anterograde
c. verigrade
d. astrograde
e. bad grade
25. What is the function of the protein coat on budding vesicles?
a. It acts as a mechanical device that helps to form the vesicle.
b. It protects the forming vesicle from osmotic shock.
c. It prevents the diffusion of harmful materials into the forming vesicle.
d. It provides a mechanism for selecting components and cargo to be carried by each vesicle.
e. a and d
26. What characteristics distinguish the classes of coated vesicles?
a. The proteins found in their coats.
b. The carbohydrates associated with their coats.
c. Their appearance in the electron microscope.
d. Their role in cell trafficking.
e. a, c and d would.
27. Which coated vesicles are NOT involved in moving materials in a retrograde direction from the Golgi complex to the ER?
a. COPII-coated vesicles
b. COPI-coated vesicles
c. clathrin-coated vesicles
d. a and b
e. a and c
28. Which of the following polypeptides would be expected to be glycosylated?
a. A histone protein bound to nuclear DNA.
b. A lysosomal enzyme.
c. COPI vesicle coat protein.
d. The SRP.
e. A cytoplasmic chaperone.
29. Which of the proteins below are selectively captured in the ER because of their ability to interact specifically with COPII proteins of the vesicle coat?
a. Membrane-bound enzymes that act at later stages of the biosynthetic pathway b. Soluble proteins floating in the lumen of the ER
c. The integral membrane protein V-SNARE, involved in docking and fusion of the vesicle with its target compartment
d. Intrinsic receptor proteins that bind soluble cargo like secretory proteins
e. a, c and d
30. What mediates the interaction between membrane proteins and the COPII-coat?
a. signal sequences in the luminal tails of ER membrane proteins
b. signal sequences in the cytosolic tails of ER membrane proteins
c. signal sequences in ER phospholipids that interact with the membrane proteins
d. signal sequences in carbohydrates on the cytosolic tails of ER membrane proteins
e. signal sequences in carbohydrates on the luminal tails of ER membrane proteins
31. What do you think will be the effect on COPI-coated vesicles if a cell is treated with GTP analogues that can bind to, but cannot trigger the active conformation of, the G-protein?
a. They will accumulate in the nucleus.
b. They will accumulate in the cytoplasm.
c. They will fuse into one giant vesicle that was seen in the cytoplasm.
d. They will decrease substantially in number in the nucleus.
e. They will decrease substantially in number in the cytoplasm.
32. COPI-coated vesicles have been implicated in the movement of in a direction.
a. Golgi-resident enzymes in a trans-to-cis direction, anterograde
b. ER-resident enzymes from the Golgi complex back to the ER, retrograde
c. Golgi-resident enzymes in a trans-to-cis direction, retrograde
d. ER-resident enzymes from the Golgi complex back to the ER, anterograde
e. b & c
33. What mechanisms have been implicated in maintaining ER-resident proteins, but not non- resident proteins, within the ER?
a. Selective export of non-resident proteins in transport vesicles
b. Degradation of proteins that drift out of the compartment in which they normally reside
c. Increased synthesis of proteins that have fallen below a threshold level in their normal compartment
d. Retrieval of escaped protein molecules, bringing them back to their normal compartment of residence
e. a and d are true.
34. Where in the Golgi apparatus does most protein sorting occur?
a. the medial cisternae
b. the TGN
c. the CGN
d. the medial golgi
e. c and d
35. If you compared proteins in the cis Golgi compartment with those in the trans Golgi compartment, you would find:
a. The proteins in both compartments are glycosylated, but there are alterations in the structure of the carbohydrate chains.
b. The proteins in the cis compartment are not glycosylated, whereas those in the trans compartment are glycosylated.
c. The proteins in the cis compartment are glycosylated, whereas those in the trans compartment are not.
d. The proteins of the cis compartment have hydrophobic sequences not present on the trans-Golgi proteins.
e. The proteins in the two compartments are identical.
36. Which is the most correct statement about the transport of membrane from ER to Golgi:
a. there is no transport from ER to Golgi
b. because the ER and Golgi are continuous with each other there is no need for membrane budding to transport material from one to the other
c. transport from ER to Golgi takes place using an intermediate called the TGN
d. membrane budding and targeting is driven by proteins in the lumen of the ER
e. transport from the ER to the Golgi occurs by budding of vesicles from the ER membranes and fusion of those vesicles with the Golgi membranes
37. How are proteins retrieved from the Golgi back to the ER?
a. They are recycled back by using protein disulfide isomerase which catalyzes cysteine- cysteine disulfide bonds
b. Chaperone proteins help fold the ER protein to bring it back to the ER
c. The SRP receptor binds SRP, triggering transport back to the ER
d. A sequence such as the KDEL amino acid sequence is recognized by receptors in the Golgi, causing the protein to be returned to the ER.
e. All of the above are true
38. All of the following could prevent a secretory protein from being secreted normally EXCEPT:
a. A mutation that results in the deletion of the N-terminal signal sequence.
b. The addition of an ER-retrieval sequence.
c. A mutation that causes addition of mannose-6-phosphate to the carbohydrate tree structure.
d. A mutation that prevents addition of N-linked oligosaccharides in the ER.
e. Glycosylation of the protein in the ER.
39. Using a mutant encoding a temperature sensitive VSV-G protein to monitor protein transport through the secretory pathway, it was observed that at restrictive temperature protein is localized in ER, but at permissive temperature it moves to it target plasma membrane. What is the likely cause?
a. At restrictive temperatures the protein is misfolded in the ER
b. At restrictive temperatures viral encoded glycoprotein cannot be produced
c. At restrictive temperatures the enzyme endoglycosidase D in golgi complex cannot trim the oligosaccharide chain on VSV-G protein
d. At restrictive temperatures the protein accumulates in the golgi complex.
e. None of the above are true.
40. Which of the listed proteins would NOT be expected to have experienced vesicular transport?
a. Trans-golgi enzymes
b. Some peroxisomal membrane proteins
c. A histone protein associated with chromatin
d. Endocytosed ligands
e. The KDEL receptor
41. What appears to be a key early step in the process of vesicle fusion to its target compartment?
a. swelling of the vesicle
b. shrinkage of the vesicle
c. tethering of vesicles to the target compartment by extended, fibrous proteins
d. a change in charge at the vesicle surface
e. dissociation many lipids from the vesicle membrane
42. Which of the molecules listed may confer specificity in the interaction between vesicles and their target compartment?
a. SNAREs
b. Rans
c. Ras
d. ARF1
e. a and b
43. When Rabs have bound to GTP, what do they do?
a. They fuse membranes directly.
b. They pass through the membrane.
c. They help tether vesicles to target membrane surfaces.
d. They denature specific membrane proteins.
e. none of the above
44. What likely determines the specificity of vesicle fusion to a target membrane?
a. only the interactions between Rabs and Rab effector tethering proteins.
b. interactions between different Rabs on the donor and acceptor compartments
c. interactions between specific combinations of multiple interacting proteins, including
Rab effector tethering proteins and Rabs, and T- and V-SNAREs
d. only the interactions between SNAREs
e. the same protein found in the membrane of the vesicle and target membranes
45. The class of coat proteins associated with ER vesicle budding is
a. COPI.
b. COPII.
c. clathrin and adapter proteins.
d. SCAP.
46. What do you think will be the effect on vesicle number if a cell is treated with GTP analogues that can bind to and activate Sar1 but cannot be hydrolyzed to GDP?
a. They will accumulate in the nucleus.
b. They will accumulate in the cytoplasm.
c. They will accumulate in the ER.
d. They will decrease substantially in number in the nucleus.
e. They will decrease substantially in number in the cytoplasm.
47. Which of the following small GTPases are NOT involved in vesicle budding or docking?
a. ARF
b. rab1
c. Sar1p
d. ALL are involved
48. COPI coat proteins mediate transport between the Golgi apparatus and the ER. What direction is this?
a. anterograde
b. enterograde
c. retrograde
d. siderograde
49. Which of the following could prevent a secretory protein from being secreted normally?
a. A mutation that causes addition of mannose-6-phosphate to the oligosaccharide structure.
b. A mutation that prevents addition of N-linked oligosaccharides in the ER.
c. A mutation that results in deletion of the start transfer sequence.
d. Addition of an ER-retrieval sequence.
e. All of the above.
50. Which of the proteins below is (are) NOT made on the RER or typically glycosylated and thus converted to glycoproteins?
a. Plasma membrane proteins.
b. Nuclear proteins.
c. Golgi resident proteins.
d. Lysosomal hydrolases.
e. b and c are not made on the RER.
51. Which of the following are made on RER-bound ribosomes?
a.Nuclear proteins
b.mitochondrial proteins
c.soluble proteins found inside lysosomes
d.secretory proteins
e. c and d
52. Referring to the picture below, loss of function mutations in which molecule(s) would result in phenotype 1, thus preventing the normal secretion of proteins out of the cell? (“Loss of function” means it is functionally absent; think of it as missing)
a. SRP.
b. Translocation channel.
c. Clathrin proteins.
d. Mutations in a or b could result in phenotype 1.
e. All of the above could result in phenotype 1.
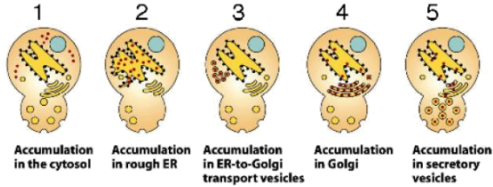
53. Which one of the following is true of material entering the cell through clathrin coated pits via the process of receptor mediated endocytosis?
a. Receptor and ligand are delivered to the nucleus to turn on gene expression.
b. Receptor and ligand always return to the plasma membrane.
c. Receptor is separated from ligand in an early endosome and the receptor can be returned to the membrane while ligand is delivered to lysosomes.
d. Receptor and ligand are internalized and delivered to the cis-Golgi for sorting.
e. Receptor and ligand are both delivered to lysosomes, but since receptors are resistant to degradation by the lysosome, they are always recycled from there to the membrane.
54. The vesicle coat protein clathrin will:
a. have an internal start transfer sequence.
b. have a man-6-phosphate modification.
c. be co-translationally inserted into the ER
d. not be glycosylated.
e. have a nuclear localization signal.
55. If a patient has a lysosomal storage disease in which all lysosomal proteins are found secreted from the cell, and those proteins have no Man-6-phosphate attached to their oligosaccharides, then the defect is most likely:
a. In the SRP receptor.
b. In the enzyme that adds man-6-p to lysosomal proteins.
c. In the man-6-p receptor
d. In the lysosomal enzyme RNAase.
e. In the enzyme that co-translationally glycosylates proteins.
56. In a cell which lacks the mannose-6-phosphate receptor for lysosomal enzymes:
a. The lysosomal enzymes would not be glycosylated and would be secreted from the cell
b. The lysosomal enzymes would be glycosylated, lack mannose-6-phosphate, and be secreted from the cell
c. All glycoproteins would have mannose-6-phosphate
d. The lysosomal enzymes would be glycosylated, have mannose-6-phosphate, and be secreted from the cell
e. None of the above.
57. Overall, cell surface area would DECREASE if:
a. The rate of clathrin-coated pit formation increased
b. The rate of clathrin coated pit formation decreased
c. The rate of constitutive exocytosis increased
d. The rate of constitutive exocytosis decreased
e. a and d are true
58. What do you think would happen if a cell were treated internally with reagents that blocked the formation of clathrin coated vesicles?
a. Receptor-mediated endocytosis would be reduced.
b. Vesicle budding from the TGN to late endosomes would be blocked.
c. Vesicle budding from the ER membranes would be blocked.
d. a and b
e. a and c.
59. All of the following are involved in COP- and Clathrin-coated vesicle trafficking except:
a. A mechanical system for regulating membrane budding
b. A mechanism for selecting cargo for inclusion
c. A mechanism for targeting the vesicle to the acceptor compartment
d. A mechanism for fusing the transport vesicle with the target membrane
e. All of the above are requirements
60. G-proteins regulate budding in all of the following ways except:
a. They can associate with the cytoplasmic side of membranes via fatty acid modifications.
b. Different types of coat protein systems can use a different G-protein
c. The G-protein covalent lipid anchor can be exposed to associate with the cytoplasmic side of membranes
d. The G-protein covalent lipid anchor is always hidden and its association with the cytoplasmic side of membranes is irreversible
e. The G-protein covalent lipid anchor can be hidden to dissociate from the cytoplasmic side of membranes
61. Which is false about the fusion process and G-proteins?
a. The class of G- proteins involved in fusion is the Rab proteins
b. When GTP is bound to Rab, fusion can occur
c. The vesicle fuses to the target membrane and maintains its membrane/protein topology
d. Following GTP hydrolysis, Rab-GDP is released from membranes into the cytosol
e. The same Rab is responsible during the fusion process for all vesicular trafficking
62. Which of the following statements about constitutive exocytosis is FALSE?
a. It occurs at a relatively constant rate.
b. It is used to secrete proteins that lack a stop transfer sequence.
c. It requires transport of proteins through the trans golgi network (TGN).
d. It requires participation of SNARE proteins.
e. It only occurs following stimulation of a cell.
63. You are tracing the path of the LDL receptor, used for cholesterol transport, from its synthesis to its destination in the plasma membrane. The order of listed compartments in which you would find it is:
I. medial Golgi
II. Trans Golgi network
III. Secretory vesicles
IV. cis-Golgi
V. Rough ER
a. V, IV, II, I, III
b. II,V, IV, I, III
c. V, IV, I, II, III
d. V, I, IV, II, III
e. V, II, I, IV, III
64. Tracking the internalization of fluorescently-labeled dextran (a charged, hydrophilic polysaccharide) is a routine method for analyzing fluid-phase endocytosis/pinocytosis. The fluorescent signal is brighter at higher pH and becomes “quenched” (reduced) at lower pH. You incubate cultured cells with culture medium containing Fl-Dextran for a period followed by a wash with Fl-Dextran-free medium. When you analyze the living cells by microscopy, you see fluorescent signal in . Over time, the signal becomes .
a. the cytoplasm; brighter
b. endosomes; fainter
c. the cytoplasm; fainter
d. endosomes; brighter
e. you would see no fluorescent signal
2024-03-07