MCB 2210 PROBLEM SET 3
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
MCB 2210 PROBLEM SET 3
1. A given protein is regulated by a protein kinase and a protein phosphatase, and is active when phosphorylated. Which would you expect to INCREASE the protein’s activity?
a. Expression of a mutant, nonfunctional kinase that blocks the activity of the cell’s normal kinases (a “dominant-negative” kinase)
b. Expression of a mutant, nonfunctional phosphatase that blocks the activity of the cell’s normal phosphatases (a dominant-negative phosphatase)
c. Expression of a mutant, hyperactive phosphatase.
d. a and b are correct.
e. b and c are correct.
2. Which statement about phosphorylation is correct?
a. The addition of phosphate from ATP ALWAYS turns the target protein “on” while their removal turns the protein “off”
b. The negative charge of the phosphate groups can attract positively charged amino acid side chains of the protein and therefore alter the overall shape of the protein and its
binding sites.
c. The addition and removal of phosphate groups from proteins occurs in response to signals that specify a change in the state of a cell.
d. Both b and c are correct.
e. a, b, and c are correct
3. Ras is a small G protein that regulates cell proliferation in response to growth factors. When Ras is in its active form, cells proliferate. The activity of Ras is carefully regulated by two other proteins: Ras GEF (guanine nucleotide exchange factor) which stimulates binding of GTP by
Ras, and Ras GAP (GTPase activating protein) which stimulates GTP hydrolysis by Ras. The activities of these regulatory proteins are, in turn, also regulated. Ras activity stimulates cell proliferation. Which of the following changes in GAP and GEF proteins might cause a cell to increase proliferation?
a. A nonfunctional GAP.
b. A permanently active GAP.
c. A nonfunctional GEF.
d. A permanently active GEF.
e. a and d are true.
4. Ran's role in regulating nucleocytoplasmic transport is based on a mechanism in which the cell maintains a nuclear concentration and a very low cytoplasmic concentration of ______.
a. low Ran-GDP, Ran-GTP
b. high Ran-GDP, Ran-GTP
c. high Ran-GTP, Ran-GTP
d. a and c
5. Of these five steps listed, what is the FOURTH step in making a nuclear protein?
a. The DNA is transcribed.
b. The mRNA is transported through the nuclear pore to the cytoplasm.
c. The protein moves through the pore into the nucleus.
d. The protein folds.
e. The mRNA is translated.
6. You have isolated the gene for a protein called nuculin that normally is transported into the
nucleus. You locate in the gene the portion of the coding sequence that codes for the nuclear
localization signal (NLS) and engineer it so that a positively charged amino acid in the nuclear
localization signal is replaced by a nonpolar amino acid, a change that disrupts the NLS and
renders it nonfunctional. You then insert the gene into cells and observe where nuculin goes after its synthesis. What is the effect, if any?
a. Nuculin will no longer be translated by the cell.
b. Nuculin will move into the ER.
c. Nuculin will move in to the nucleus as it usually does.
d. Nuculin will localize to mitochondria.
e. None of the above is a correct statement.
7. Which of the following is FALSE about nuclear localization signal and nuclear export signal?
a. Nuclear export receptors recognize the NES to be brought back to the cytoplasm.
b. Nuclear import receptors recognize the NLS to be brought into the nucleus.
c. The NES and NLS are specific sequences that drive transport in opposite directions.
d. The NES and NLS are specific sequences that drive transport in the same direction.
e. Movement is regulated through the nuclear pore complex with the aid of G-proteins.
8. Which of the following is not a function of the nuclear lamina?
a. It provides mechanical support for the nuclear envelope.
b. It is a point of attachment for the enzymes that synthesize histone.
c. It serves as an attachment site for chromatin fibers at the nuclear periphery.
d. It binds to ribosomes that are conducting protein synthesis.
e. b and d
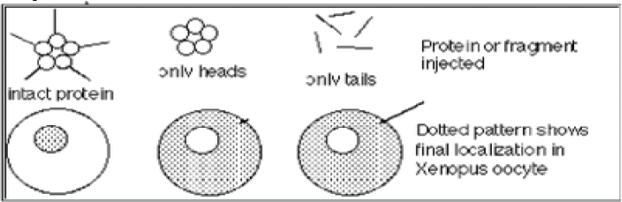
9. You isolated a protein and tagged it with a fluorescent probe. You microinjected the protein
into the cytoplasm of a Xenopus oocyte and observed the localization of the protein as shown in the diagram. The stippled (dotted) region is the area that appears fluorescent. What is the correct interpretation of the experiment?
a. Tail is necessary but not sufficient for the protein to be transported into the nucleus.
b. Head is necessary and sufficient for the protein to be transported into the nucleus.
c. The tail is neither necessary nor sufficient for import into the nucleus.
d. Neither tail nor head alone is sufficient for import into the nucleus.
e. a and d
10. Which of the following things can be moved either in, out, or in both directions through nuclear pores?
a. mRNAs
b. snRNAs
c. ribosomal subunits
d. tRNAs
e. all of the above
11. What happens to Ran-GTP after it has been transported to the cytoplasm bound to an importin?
a. Bound GTP is hydrolyzed to GDP.
b. Bound GTP is hydrolyzed to GMP.
c. Ran-GDP is released from the importin.
d. Ran-GTP binds to the importin subunit.
e. a and c are correct.
12. After transport into the nuclear compartment, what interacts with the importin-cargo complex and what effect does it have on the complex?
a. Ran-GTP, causes the importin-cargo complex to disassemble
b. Ran-GDP, causes the importin-cargo complex to disassemble.
c. Ran-GTP, causes the importin-cargo complex to be destroyed.
d. Ran-GDP, causes the importin-cargo complex to be destroyed.
e. Ran-GTP, causes the importin-cargo complex to be stabilized
13. Which protein serves as an accessory protein that typically resides in the cytoplasm where it promotes the conversion via hydrolysis of Ran-GTP to Ran-GDP?
a. Ran-GTP
b. Ran-GEF
c. Ran-GAP
d. Ran-GDP
e. Ran-GMP
14. Which of the following correctly describes components that interact to successfully complete the import of cargo proteins from the cytoplasm to the nucleus?
a. cargo—importin—Ran-GTP complex
b. cargo—exportin—Ran-GDP complex
c. cargo—exportin—Ran-GTP complex
d. cargo—importin—Ran-GDP complex
e. None are correct.
15. What would you expect to happen if you massively overexpressed a very active RanGEF in the cytoplasm, such that the normal activity of RanGAP was completely overridden?
a. Ran would not be recycled back to the nucleus by Ntf2.
b. Any exported Cargo/Exportin/RanGTP complexes would abnormally persist in the cytoplasm due to the excess RanGTP there.
c. Import of proteins would be accelerated.
d. Importins returning to the cytoplasm could not bind cargo proteins because they would remain bound to the excess RanGTP present there.
e. a, b and d are correct.
16. Of these five steps listed, what is the FOURTH step in making a nuclear protein?
a. An importin binds to the NLS and moves the protein through the nuclear pore into the nucleus.
b. The mRNA encoding for the nuclear protein is transported through the nuclear pore to the cytoplasm.
c. The mRNA is translated.
d. The protein folds.
e. The importin/protein complex encounters Ran-GTP and falls apart.
17. Competition experiments have been used to determine that there are different kinds of
exportins for each species of RNA. You are studying importins for proteins. You translate in
vitro two different nls-containing proteins. Protein A is fused to GFP, while the other (protein B)
is left unlabeled. Which statement below correctly describes results and interpretations that you might obtain from observing the behavior of these proteins injected into the cytoplasm of oocytes?
a. Injecting an excess of protein B would not affect import of protein A into the nucleus if both use the same importin.
b. Injecting an excess of protein B would decrease import of Protein A into the nucleus if both use the same importin.
c. Injecting an excess of Protein B would not alter import of Protein A into the nucleus if both use different importins.
d. a and b.
e. b and c.
18. If gold particles of varying size are injected into the cell, they can be seen to enter what structure? What technique was used to visualize these events?
a. nuclear pores, electron microscopy
b. nuclear pores, light microscopy
c. directly through the nuclear envelope membranes, electron microscopy
d. directly through the nuclear envelope membranes, light microscopy
e. ribosomes, electron microscopy
19. Your investigations discover a protein that is normally present in the cytoplasm, but when you treat cells with a hormone, it moves to the nucleus. What is the most likely explanation?
a. A nuclear targeting signal is post-translationally added onto the protein to target it to the nucleus.
b. The nuclear localization signal is initially buried in the protein until, after hormone treatment, the protein changes its conformation thereby exposing the NLS and allowing transport to the nucleus.
c. After hormone treatment, the protein unfolds, enters the nucleus and then refolds.
d. The protein is allowed to enter the RER after hormone treatment to enable it to move to the nucleus.
e. Hormone treatment activates the normal pathway of co-translational import of proteins into the nucleus.
20. You take the normal NLS and engineer its fusion to a non-nuclear protein like ovalbumin.
Next, you also fuse GFP in-frame with this new protein and inject it into the cytoplasm of the
cell and use microscopy to monitor what happens to the protein. What would you expect to see?
a. GFP would be visualized in the nucleus of the cell.
b. GFP stays cytoplasmic and is excluded from the nucleus.
c. Ovalbumin is immediately denatured, so you see no GFP signal.
d. Ovalbumin is immediately degraded, so you see no GFP signal
e. d and e
21. What happens to Ran-GTP after it has been transported back to the cytoplasm following its release of cargo from the importin α/β molecule?
a. It is hydrolyzed to Ran-GDP.
b. It is hydrolyzed to Ran-GMP.
c. The Ran-GDP is released from the importin β subunit.
d. a and c
e. It binds to the importin α subunit.
22. How can an importin subunit be transported back to the cytoplasm? (See Pemberton and Paschal, 2005 slide in nuclear transport lecture notes)
a. Importin β subunit can diffuse back through the nuclear pore complex bound to Ran- GTP.
b. Importin a can be transported back to the cytoplasm by Cas, an exportin, in a trimeric complex that included Ran-GTP.
c. It can denature in the nucleus, pass through the membrane and renature in the cytoplasm.
d. It can be transported back to the cytoplasm by another importin, importin γ.
e. a and b are correct.
23. Your undergraduate honors project is to isolate a new protein from the nucleus of the cell. In order to test that you got the right protein, you decide to inject it into the cytoplasm of frog eggs and see if it is transported into the nucleus. You are relieved to find that this experiment works, and your advisor breaks out the champagne (which is probably a violation of some university policy). Next, you decide to isolate the domain containing the targeting signal by taking 5 equal sized pieces of the protein and testing them individually. You find that no matter which piece you test on its own, none are transported into the nucleus. However when you take pieces 2 and 3 and mix them, both pieces are imported. No other combination works except those containing pieces 2 and 3. Your conclusion is:
a. There is no nuclear targeting signal on this protein.
b. 2 and 3 are both necessary for targeting.
c. 2 and 3 both contain the same targeting signal.
d. Neither 2 nor 3 alone are sufficient for targeting.
e. b and d are correct.
24. Once a protein-exportin-RanGTP complex has reached the cytoplasm, all of the following occur except:
a. GTP is hydrolyzed.
b. The protein is released.
c. Ran-GDP and exportin separately return to the nucleus.
d. Ran-GDP is directly rephosphorylated to GTP on the nuclear side.
e. All of the above occur
25. Which of the following is FALSE about the Ran G-protein?
a. Ran is present in its inactive form (GDP) in the cytoplasm.
b. Importin receptor recognizes NLS on protein to be imported, the complex moves to the nucleoplasm, and is then recognized by RanGTP due to presence of ranGEF.
c. Protein cargo released in to the nucleus so the receptor and RanGTP can go back to the cytoplasm.
d. RanGTP does not encounter GAP when it returns to the cytoplasm.
e. Ran is present in its active form (GTP) in the nucleus.
26. If a nuclear localization signal were added to the gene encoding a lysosmal protein, where would the protein likely be found?
a. Nucleus
b. Cytoplasm
c. Mitochondria
d. Lysosomes
e. Secreted from the cell.
27. Which of the following is true regarding the Endoplasmic Reticulum?
a. site of co-translational glycosylation of secretory proteins
b. site of phospholipid synthesis
c. site of GPI lipid anchoring of membrane proteins
d. site of synthesis of oligosaccharides for co-translational attachment to proteins
e. all of the above are true
28. Which one of the following is not a function of the ER?
a. Site of membrane and secretory protein synthesis.
b. Site of Ca2+ storage.
c. Site of lipid synthesis.
d. Detoxifying enzymes in liver.
e. Site of degradation of phagocytosed bacteria.
29. Which of the proteins below is(are) not made on the RER, glycosylated and thus converted to glycoproteins?
a. transmembrane plasma membrane proteins
b. soluble lysosomal proteins
c. mitochondrial enzymes
d. proteins of the extracellular matrix
e. all of the above
30. Which step listed is not true about protein synthesis on the rough ER?
a. The hydrophobic signal peptide emerges from the ribosome.
b. The signal peptide binds to the hydrophobic site on the ribosome.
c. SRP is bound to the signal peptide.
d. The ribosome with a bound SRP binds to the SRP receptor on the rough ER.
e. The synthesized protein translates through the pore of the rough ER.
31. All of the following are essential components of the complex that directs a nascent protein into the lumen of the RER except:
a. Ribosome
b. SRP
c. Cis golgi
d. Translocation channel
e. All are essential components
32. What are the two sites within a cell at which protein synthesis is generally thought to occur? a. cytosolic surface of RER and cisternal surface of RER
b. cisternal surface of RER and free ribosomes
c. cytosolic surface of RER and free ribosomes
d. cytosolic surface of RER and cytosolic surface of SER
e. free ribosomes and cytosolic surface of SER
33. What effect does the docking of the SRP/ribosome/growing polypeptide chain complex to the SRP receptor have on protein synthesis?
a. The SRP will release from the ribosome.
b. Protein synthesis ceases temporarily.
c. Protein synthesis resumes.
d. The ribosome releases the growing polypeptide chain.
e. a and c are correct.
34. All of the following hold the SRP-ribosome-nascent polypeptide complex onto its binding site on the RER membrane except:
a. The interaction between the SRP and the SRP receptor.
b. Its interaction with sphingomyelin.
c. The interaction between the ribosome and the protein-lined membrane channel.
d. The interaction between the ribosome and the translocon.
e. a, c and d
35. Hydrophobic alpha-helices that become transmembrane domains, and that interact with the translocon machinery to determine their orientation in the membrane are also known as:
a. topiary sequences
b. tongue and groove sequences
c. touchy feely sequences
d. topogenic sequences
e. totally cool sequences
36. What poorly understood event occurs during the docking of the ribosome to the translocon and the subsequent release of SRP and SRP receptor from the ribosome and translocon?
a. cleavage of the signal peptide
b. nuclear import
c. nuclear export
d. a halt in protein synthesis
e. GTP binding and hydrolysis by both the SRP and SRP receptor
37. Many integral membrane proteins have a single segment in the nascent chain that serves as both a signal sequence for binding SRP and a sequence that codes for insertion into the lipid
bilayer. What is it called?
a. stop-transfer sequence
b. signal sequence
c. signal peptide
d. internal start transfer or signal-anchor sequence
e. stop-signal sequence
38. Stop-transfer sequences typically include .
a. at least 15 continuous hydrophobic or uncharged amino acids
b. at least 15 continuous hydrophilic or charged amino acids
c. at least 15 continuous hydrophobic or charged amino acids
d. at least 15 continuous hydrophilic or uncharged amino acids
e. a and d
39. How is the orientation of membrane proteins in the membrane thought to be accomplished?
a. After synthesis, an enzyme orients the protein properly.
b. During synthesis, the translocon inner lining orients the nascent polypeptide so that the positive charged amino acids adjacent to the end of the TM domain face the cytosol.
c. During synthesis, the translocon inner lining orients the nascent polypeptide so that the negative charged amino acids adjacent to the end of the TM domain face the cytosol.
d. During synthesis, the translocon inner lining orients the nascent polypeptide so that the positive charged amino acids adjacent to the end of the TM domain face the mitochondria.
e. After synthesis, the translocon inner lining orients the nascent polypeptide so that the positive charged amino acids adjacent to the end of the TM domain face the cytosol.
40. What would be the effect on synthesis of an ER-targeted protein if you overexpressed a
mutant SRP that was able to bind to the signal sequence and the ribosome, but could not interact properly with the SRP receptor?
a. The protein would end up full-length in the cytoplasm
b. The protein would enter the ER normally
c. Much more of the protein would be produced than normal
d. Synthesis of the protein would be inhibited
e. b and c are correct
41. What would be the effect on synthesis of an ER-targeted protein if you overexpressed a mutant SRP that was unable to bind to, and interact properly with, the signal sequence and ribosome?
a. The protein would enter the ER.
b. The protein would end up a cytosolic protein.
c. The protein would be secreted from the cell.
d. Synthesis of the protein would be inhibited.
e. b and c are correct.
42. If free ribosomes in the presence of RER vesicles with a mutant, nonfunctional SRP receptor that cannot bind the SRP, are placed in a test tube with mRNA for a protein with an n-terminal signal sequence, and everything else needed synthesize proteins, along with the addition of SRP, where are the proteins found after their production?
a. There are no full-length proteins made.
b. Inside the RER-derived vesicles.
c. Floating free in the aqueous solution in the test tube.
d. Inside microsomes.
e. None of the above.
43. You isolated cellular components to study the transport of proteins in a cell. After a lot of hard work, you take a vacation, but you forget to write down the components of your final preparation. So, you put some of your preparation in a test tube, and then add mRNA for a secretory protein, along with tRNAs, amino acids, ATP. You find that the mRNA is completely translated, and the new protein is sensitive to protease, if added. Your preparation has:
a. Ribosomes, SRP, and typical microsomes.
b. Ribosomes, SRP, and microsomes containing a mutant SRP receptor.
c. Ribosomes and typical microsomes, but no SRP.
d. Ribosomes, SRP, but no microsomes.
e. SRP and microsomes, but no ribosomes.
44. You are studying two proteins, with the goal of understanding how they might be targeted
to the ER. Protein A has the same molecular weight when it is synthesized in vitro without rough ER (RER) vesicles or SRP, as it does when synthesized in the presence of both RER vesicles and SRP. Protein B is apparently 10- 15 amino acids shorter when synthesized in the presence of
vesicles and SRP than without them. What statement about the results is true?
a. Protein B must use an internal start transfer sequence.
b. Protein B must use an N-terminal signal sequence.
c. Protein A must use an N-terminal signal sequence.
d. a and b.
e. b and c.
45. The following are involved with synthesis of a secreted protein. Which is the FOURTH step of the five listed?
a. The SRP dissociates from the ribosome and start transfer sequence
b. The SRP binds to the start transfer sequence.
c. The signal (start transfer) sequence is removed by signal peptidase.
d. The SRP binds to SRP receptor
e. Protein synthesis halts temporarily
46. One of the hallmarks of co-translational import of proteins into the ER is the insensitivity of proteins to protease if translation occurs in the presence of SRP, SRP receptor and RER vesicles. You are studying synthesis of what you think is a protein that resides in the extracellular matrix (secreted from the cell). You do the classic protease experiment and find that the protein is not
degraded at all by protease. Which statement below is correct?
a. The protein most likely uses an internal start transfer sequence.
b. The protein uses an N-terminal signal sequence.
c. The protein most likely contains no N-terminal signal sequence or internal start transfer sequences.
d. The protein likely contains multiple start transfer and stop transfer sequences.
e. None of the above can account for this behavior.
47. In those cases where post-translational transport of proteins into the ER lumen occurs, how do proteins enter the ER?
a. They go through the same channels as the proteins that enter co-translationally.
b. They are held in an unfolded conformation by chaperones so they may pass through translocons.
c. They pass into the ER lumen in their final tertiary conformation.
d. a and b are correct.
e. a and c are correct.
48. If ER-derived microsomes are placed in a test tube along with free ribosomes, mRNA for a protein that uses an n-terminal signal sequence, and everything else needed to synthesize proteins, together with a mutant, nonfunctional SRP that cannot bind the signal sequence, where would the proteins be found after their production?
a. There would be no full-length proteins made.
b. Inside the RER-derived vesicles.
c. Floating free in the aqueous solution in the test tube.
d. Tethered to the outside of the microsomes.
e. None of the above.
49. What is the purpose of molecular chaperones like BiP and calnexin?
a. They associate with unfolded and misfolded proteins and help them regain their native structure.
b. They associate with unfolded and misfolded DNAs and help them regain their native structure.
c. They associate with unfolded and misfolded RNAs and help them regain their native structure.
d. They associate with unfolded and misfolded carbohydrates and help them regain their native structure.
e. They transport secretory proteins into secretory vesicles.
50. Where are the signal peptidase and oligosaccharyltransferase located?
a. Embedded in the RER membrane facing the cytosol.
b. Floating in the lumen of the RER.
c. Embedded in the RER membrane facing the lumen.
d. Associated with the translocation channel.
e. In the Golgi apparatus
f. c and d are correct.
51. If you engineered a new protein by taking the gene for a single pass transmembrane protein that has an n-terminal signal sequence and remove the stop transfer sequence from the middle of the gene, what is a possible destination of the new protein?
a. It would be found in the cytoplasm.
b. It would be glycosylated and transported to mitochondria.
c. It would be found in the nucleus
d. It would be found as a single pass membrane protein in the plasma membrane.
e. None is a possible destination.
52. If you inserted into a cell a gene that codes for a protein that normally enters the nucleus because it has a c-terminal NLS, but you added an N-terminal ER signal sequence to it, what would you expect the FINAL destination of the protein to be?
a. Ribosome.
b. Nucleus.
c. Mitochondrial matrix
d. Secreted from the cell.
e. Cytoplasm.
53. If a nuclear localization signal were added to the gene encoding a plasma membrane protein, where would the protein likely be found?
a. Nucleus.
b. Plasma membrane.
c. Cytoplasm.
d. Lysosomes.
e. Secreted from the cell.
54. What does UGGT do if it encounters a correctly folded glycoprotein?
a. It degrades the oligosaccharide chain.
b. It binds via hydrophobic interactions and adds a single glucose back to the recently trimmed oligosaccharide.
c. It adds a single mannose back to the recently trimmed oligosaccharide.
d. It degrades the protein.
e. None of the above are correct.
55. What are the differences between ribosomes that make secretory proteins and those that make proteins intended for the cytosol?
a. The ribosomes that make secretory proteins are smaller.
b. The ribosomes that make cytosolic proteins are larger
c. There are no differences between them.
d. The ribosomes that make secretory proteins are denser.
e. a and b are correct.
2024-03-06