CHEM 100/100G Molecules that Changed the World 2020
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
CHEM 100/100G
SUMMER SCHOOL 2020
CHEMISTRY
Molecules that Changed the World
SECTION A: Answer QUESTION ONE
QUESTION ONE (answer ALL parts a-o):
(a) Match these terms with their definition/description. Write the term that matches the description for each question number in your answer booklet.
. atom
. glyphosate
. molecule
. tin
i) Smallest particle still characterising a chemical element.
ii) The smallest unit of a chemical compound - a group of atoms chemically bonded together and acting as a unit.
iii) What Napolean’s buttons were made of – in low temperatures convert from a metallic state to a powder.
iv) A currently-used herbicide produced by Monsanto and sold as Round Up. (0.5 marks each, 2 marks total)
(b) DDT and Thalidomide are both molecules that many would argue “changed the world”. Choose ONE of these and briefly explain why it was so important to society and what human health issues and/or environmental impacts your chosen molecule has. (3 marks)
For parts (c)-(g) write the correct answer (A, B, C or D) in your answer booklet alongside the question number.
(c) Which of these statements on bacteria is FALSE:
A: Bacteria have a cell wall
B: Bacteria reproduce quickly, through binary fission
C: Bacteria are more complex than animal cells
D: Bacteria can be divided into two types; Gram positive and Gram negative (1 mark)
(d) Natural products are compounds that are isolated from natural sources. Which of these are known sources for natural products:
A: Microbes
B: Flowers
C: Trees
D: All of the above (1 mark)
(e) After Alexander Fleming made the initial discovery of penicillin in 1928, he did not pursue further research on it, for what reason?
A: He discovered it was very unstable and did not believe an unstable compound would be of great clinical use
B: He discovered the mould broth he made (which contained penicillin) was toxic
C: He did not think his discovery was important or impactful
D: He did not think the world was ready for penicillin (1 mark)
(f) Which of these statements on the development and use of penicillin is FALSE?
A: Florey and Chain were the first to develop a way to obtain enough penicillin to demonstrate its efficacy
B: American pharmaceutical companies developed techniques to produce large quantities of penicillin during World War 2
C: After the war, penicillin was unrestricted and could be brought over-the-counter D: Bacterial strains resistant to penicillin have only started occurring in the last 20 years (1 mark)
(g) Drawn below is the structure of penicillin. What structural feature is NOT essential for its biological activity against bacteria?
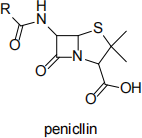
A: β-lactam ring
B: Bicyclic ring system
C: Free carboxylic acid
D: R group (1 mark)
(h) Over 1 billion tons of plastics is produced since 1950’s. Plastic garbage has become a widely recognized source of pollution.
i) Name four plastics that are low in cost and widely used. (1 mark)
ii) What are the three current methods of plastic waste disposal? (1 mark)
iii) Comment on New Zealand’s current plastic waste disposal approaches. (3 marks)
(i) According to the International Union of Pure and Applied Chemistry (IUPAC), what is the definition of alcohol? (1 mark)
(j) According to the IUPAC definition, which of the following molecule(s) can be classified as alcohol?
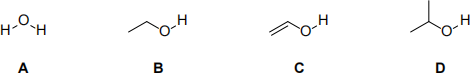 (2 marks)
(2 marks)
(k) Draw the structure of ethanol. (1 mark)
(l) Is ethanol a primary, secondary or tertiary alcohol? (1 mark)
(m)What is an alloy? (1 mark)
(n) Purity of gold is measured in ‘carats’ and is expressed by how many parts of it is pure gold. An 18ct gold will contain how many parts of pure gold and alloy? (1 mark)
(o) Gold is the most malleable and ductile pure metal known, and both these physical properties are important in the use of gold for adornment and jewellery. How do you differentiate between these two properties? (3 marks)
Total = 25 marks
SECTION B: Answer ANY THREE questions from QUESTIONS TWO-FIVE
QUESTION TWO: Penicillin (answer ALL parts a-e):
(a) List two classes of biomolecules that drugs interact with, in the body. (2 marks)
(b) Before the discovery of penicillin, there were a number of scientists who greatly contributed to the development of medicinal chemistry and our understanding of disease, including:
. Edward Jenner
. Louis Pasteur
. Robert Koch
. Joseph Lister
. William Perkin
. Paul Ehrlich
Choose TWO of these scientists and briefly describe their contribution. (3 marks each, 6 marks total).
(c) Prontosil (pictured below) is an example of a sulfa drug, the first class of drugs accepted as clinically useful in the treatment of bacterial infections.
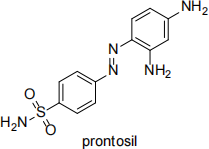
i) Prontosil is a prodrug. Give the definition of a prodrug. (2 marks)
ii) Briefly describe the mechanism of action of prontosil. (3 marks)
(d) The structures of general penicillin and D-Ala-D-Ala are given below:
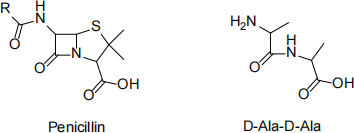
i) Describe the mechanism of action of penicillin with reference to the structure of D-Ala-D-Ala. (6 marks)
ii) One of the ways that bacteria try to become resistant to pencillins is by producing β-lactamase enzymes. What does β-lactamase do to penicillins? (2 marks)
iii) State one thing that can be done to stop the effects of β-lactamase. (1 mark)
(e) There are other classes of antibiotics other than sulfa drugs and penicllins, both mentioned above.
i) Cephalosporins (pictured below) are another class of antibiotics. By referencing the structure of the natural product 7-ACA, explain why cephalosporins are considered to be “semi-synthetic”.
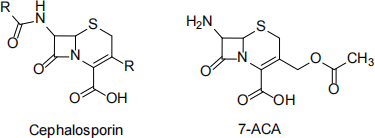 (2 marks)
(2 marks)
ii) Name one other class of antibiotic. (1 mark)
Total = 25 marks
QUESTION THREE: Nylon (answer ALL parts a-e):
(a) Please answer the following multiple choice questions focused on “Nylon”. Write the correct answer (A, B, C or D) in your answer booklet, alongside the question number.
i) Nylon 6,6 is formed by:
A: Condensation polymerisation
B: Addition polymerisation
C: Free radical polymerisation
D: Ring opening polymerisation
ii) In which company's labs was nylon invented?
A: Dow Chemicals
B: Monsanto
C: DuPont
D: Resene
iii) Who invented nylon?
A: Charles Stine
B: Julian Hill
C: Hermann Staudinger
D: None of the above
iv) What mass-produced consumer product was nylon first used for?
A: Tooth brushes
B: Lingerie
C: Mosquito netting
D: Ropes
v) Which of the following bonds is present in nylon?
A: Ether
B: Peptide
C: Phosphodiester
D: 1,4-β linkage
vi) Why does nylon make such good fibres?
A: The molecules are long and skinny
B: It is water resistant
C: The hydrogen bonding leads to high strength
D: Termites will not eat nylon
vii) What would nylon 6,10 look like?
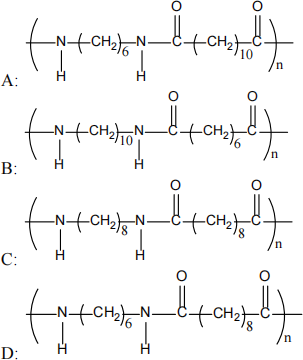
viii) Hydrogen bonding in nylon occurs between
A: N-H and C=O groups
B: N-H groups and C atoms
C: C=O groups and C atoms
D: C=O groups and double bonds
ix) Which of the following is not a nylon cousin?
A: Nomex
B: Kevlar
C: Lycra
D: Teflon
x) What is the melting point range of nylon 6,6 ?
A: 0~100°C
B: 100~200°C
C: 200~300°C
D: 300~400°C (1 mark each, 10 marks total)
(b) This year marks the 85th anniversary of the invention of nylon. Nylon has changed our world. You can find it in stockings, socks, umbrellas, raincoat, toothbrushes in our day- to-day lives.
i) What are the names of the two chemicals used to manufacture nylon? (2 marks)
ii) What is the significance of nylon invention? (3 marks)
(c) Plastics offer a wide range of properties and flexibility of designs not found in metals.
Name three basic characteristics of plastics which are lacking in ceramics and metal. (3 marks)
(d) The annual global production of Polyethylene is around 80 million tonnes. The two most common uses of polyethylene are for packaging applications, such as Ziploc bags, clingwrap, and milk bottles. Among many varieties of polyethylene, the low-density polyethylene (LDPE) was invented in 1939 and the high-density polyethylene (HDPE) was produced in 1956 by the Ziegler–Natta catalyst in 1956.

i) What are the differences in molecular structures between LDPE and HDPE? (2 marks)
ii) Which of these (LDPE or HDPE) do you think would be more appropriate for use in making milk bottles? Why? (2 marks)
(e) Name three common thermoplastic processing technologies and give one example of a commodity made by each of these processes. (3 marks)
Total = 25 marks
QUESTION FOUR: Alcohol (answer ALL parts a-f):
(a) Humans can tolerate the consumption of ethanol but not methanol.
i) The primary pathway to break down methanol inside our body involves two enzymes. Name the two enzymes, and briefly describe the roles of these two enzymes and the fate of the methanol. (5 marks)
ii) Why is methanol toxic to humans? (2 marks)
iii) Describe the factors that determine how fast ethanol is absorbed into our body. (2 marks)
(b) Ethanol is used as a renewable energy source. Describe schematically the carbon cycle involving ethanol as a biofuel. (4 marks)
(c) Ethylene glycol is used as an antifreeze. Describe how ethylene glycol can lower the freezing point of water. (4 marks)
(d) Alcohol is used as a disinfectant. A mixture of 70% alcohol and 30% water is more effective than 100% alcohol. Explain the reasoning behind this observation. (4 marks)
(e) A breathalyzer is an instrument that is used by law enforcement officers to check the blood alcohol concentration in breath during roadside tests.
i) Potassium dichromate is an essential component in a breathalyzer. Briefly describe its function. (2 marks)
ii) Silver nitrate is an essential component in a breathalyzer. Explain its function. (1 mark)
(f) By using the boiling point diagram below, if one starts off with a solution mixture that contains 20% solution A and 80% solution B, what would be the proportion of solution A and solution B in the distillate after one round of distillation at around 78 degrees?
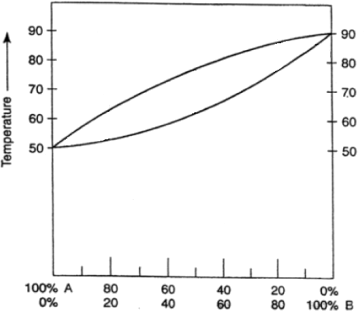
(1 mark)
Total = 25 marks
QUESTION FIVE: Gold (answer ALL parts a-e):
(a) Please answer the following multiple-choice questions focused on “Gold” . Write the letter of the correct answer in your answer booklet alongside the question number.
i) Which one of the following is the heaviest element?
A: Iron
B: Lead
C: Gold
ii) Which one of the following is the softest element?
A: Gold
B: Tungsten
C: Chromium
iii) The transmutation of base (common) metals into gold is called?
A: Philosopher’s stone
B: Alchemy
C: Alchemist
iv) The inertness of gold makes it soluble only in:
A: Hydrochloric acid
B: Nitric acid
C: Aqua regia
D: none of the above
v) A gold-containing drug named Auranofin has been primarily in use for the treatment of:
A: Rheumatoid arthritis
B: Infectious Diseases
C: Alzheimer's disease (1 mark each, 5 marks total)
(b) For each of the following statements, choose the correct answer TRUE or FALSE and write this in your answer booklet alongside the question number.
i) Gold was first used as money around 6000 BC.
ii) Relativistic effects result in the yellow colour of gold.
iii) Gold reacts readily with air (oxygen) or water.
iv) Economic value of gold-bearing ore depends on concentration of gold and cost of extraction.
v) The Egyptians were the first to develop hydraulic mining using high pressure
water to break up rock and expose gold-bearing earth.
vi) Gold is used in electronics due to its high thermal conductivity.
vii) Gold is used in space technology due to its high resistance (ohm m).
viii) Material used for a dental restoration must be reactive towards fluids in the oral (mouth) environment.
ix) Nanoparticles are discrete particles of an element or material that are sized between 1 and 10 nm (1 nm = 1 x 10-9 m).
x) Gold nanoparticles (AuNP) are prepared by reduction of HAuCl4 in water using citrate (reducing agent). The deep-red colour of AuNP colloids in water and glasses reflects the surface plasmon band. (1 mark each, 10 marks total)
(c) Define catalysts and give three emerging uses of gold catalysts. (5 marks)
(d) In its processing, gold (and silver) present in finely ground particles are dissolved into an alkaline aqueous solution containing potassium cyanide. Why is the use of cyanide one of the controversial aspects of gold processing? (2 marks)
(e) Explain what alluvial (placer) deposits of gold are and state their features. (3 marks)
Total = 25 marks
2024-02-03