CHEM224 2023 SI Session #10
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
CHEM224 2023 SI Session #10
Learning Objectives: By the end of this session, students should be able to:
● Differentiate between the reactivity ofthioethers and ethers
● Explain how we can use silyl-ethers as protecting groups
● Explain the synthesis routes and relative reactions of epoxides
Section 1. Thioethers
1. Use the following thioether to answer the questions below
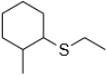
a. Provide the common and IUPAC names of this compound
b. Synthesize this compound from 2-methylcyclohexan-1-thiol, using any additional reagents you need
2. EXAM QUESTION (modified): What is the result of the following reaction? Why is this different from when we have a normal ether?
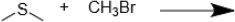
3. The product in question 2 reacts with PhMgBr. Draw the result of this reaction, and supply a mechanism to support your answer.
Section 2. Silyl Ethers and Protecting Groups
4. EXAM QUESTION (modified): Provide the reagents required to complete the chemical transformation.

Section 3. Making Epoxies
5. Synthesize the following epoxide using the specified methods. Use any additional reagents you need
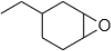
a. Via a peroxyacid
b. Via halohydrin cyclization
6. Provide a mechanism for (b) above.
7. Draw the product of the following reaction.
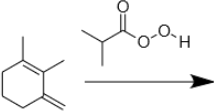
Section 4. Breaking epoxides (acid & base)
8. The following reaction has two potential products (which are shown). Which is produced in the highest abundance? Explain your reasoning using a mechanism
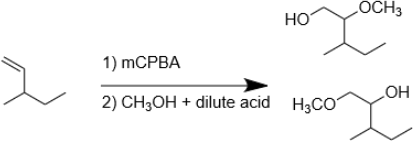
9. Draw the mechanism of the following reaction. Explain, using a mechanism, why we get the product shown.
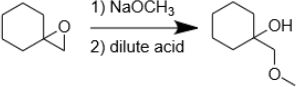
10. Integrated Synthesis Practice: Provide the reagents required to perform the following chemical transformation.
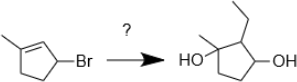
2024-01-22